Ethical Pharmaceutical Business
Mainstay Products
Leading the Onychomycosis Market in Both Topical and Oral Treatments NAILIN and LUCONAC
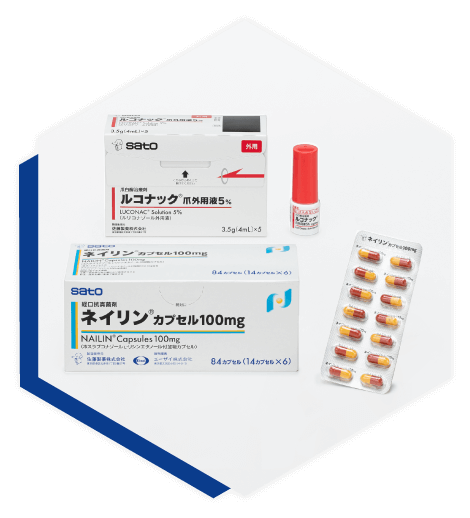
Our ethical pharmaceuticals business is driven by drugs related to the treatment of onychomycosis in the dermatology field. In 2016, Sato launched a topical onychomycosis treatment, LUCONAC Solution 5%, and in 2018, NAILIN Capsules 100mg, an oral onychomycosis treatment. Further, we are in the process of obtaining approval for LUCONAC in Singapore and expanding globally. As the only pharmaceutical company in Japan with both topical and oral formulations, Sato will continue to lead the market for onychomycosis treatments.

Taking Top Share in the External Topical Anesthetic Market EMLA Series

In 2012, Sato launched EMLA Cream, Japan’s first effective topical anesthetic pain- reduction therapy for dermatological laser radiation treatment. In 2015, application was expanded for relief of pain during injections and puncture of intravenous indwelling needle. In 2017, the EMLA Patch, a one-touch procedure, was launched as an additional dosage form. In 2018, EMLA took the top share of the domestic external anesthetic market (based on NHI price).

Contributing to Patient QOL with a Highly Original Product Line
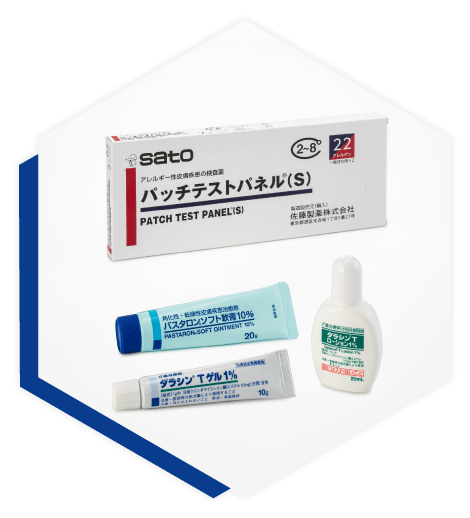
Sato is also developing other unique products, primarily in the field of dermatology. PASTARON, a urea preparation, has been expanded to include a variety of products, and has been in use in the medical field for many years. We also address a wide range of needs in the medical field with products including the DALACIN series, an acne remedy, and PATCH TEST PANEL (S), a diagnostic agent for allergic dermatitis, contributing to improving patient quality of life.

Marketing
-
Efforts as a Leading Company in Onychomycosis, and Promotional and Educational Activities
As the only pharmaceutical company in Japan that has both topical and oral formulations for the treatment of onychomycosis—NAILIN and LUCONAC—Sato Pharmaceutical offers a variety of lectures and provides a wide range of information utilizing digital media.

-
Creating Sato Pharmaceutical’s Unique Web Seminars
We have created Sato Derma Online (SDO) as a new platform for distributing Sato Pharmaceutical’s unique web seminars. We distribute useful information to dermatologists, primarily around onychomycosis. There were approximately 3,000 physicians registered as of March, 2022. About 10 seminars are held each month, each of them viewed by between about 200 and 300 doctors.

Educational Activities for Patients Using Digital Media
Sato has launched a website about onychomycosis. The goal is to have more patients visit medical institutions for diagnosis and a complete cure by giving them a correct understanding of the impact of onychomycosis on quality of life, such as the walking function.
Research and Development
Cutting-edge Efforts Aimed at Creating New Drugs
The Shinagawa R&D Center, located in Shinagawa-ku,
Tokyo, is lined with facilities that include the New Research
Wing, the Formulation Research Wing and others. Here, four
departments—the Research Planning Office, the Drug Discovery
Research Department, the Ethical Pharmaceutical Research
Department and the Formulation Research Department—
conduct research aimed at creating new drugs.

Utilizing Cutting-edge Technologies to
Quickly Respond to the Need for New
Drugs the Times Demand
The Shinagawa R&D Center conducts the research and development activities needed to produce Sato’s ethical pharmaceuticals, OTC drugs, quasi-drugs, cosmetics, and foods for specified health uses. The R&D Center pursues research into high value-addition products that are always based on the needs of consumers. Currently, it focuses on four main themes: ocular disease, wound healing, antifungal agents and sarcopenia. Active research is underway to meet the needs of the medical field, including new compound synthesis, biological screening, and pharmacological and pharmacokinetic assessment.
-
Table 1: Status of products in development and currently under investigation
Development code Treatment Active ingredient Development stage SKN-14 Acne New active ingredients
(license in)Phase I clinical study SKN-15 Viral skin disease New active ingredients
(license in)Phase I clinical study -
Table 2: Status of products developed internally
Disease area Research status ocular disease Non-clinical development research Wound healing Lead compound optimization antifungal agents Lead compound exploration Sarcopenia Drug discovery, target identification
Description of Main Research
-
Joint Research with
Specialist Institutions
Joint research with Kyushu University
Sato proactively engages in joint research into drug discovery with companies, universities, and research institutes in Japan and overseas. In addition to drug discovery activities targeting lipid metabolism- related enzymes with Kyushu University, we have established the Advanced Research Chair in Wound Healing in collaboration with Juntendo University, and are conducting joint research in the field of ophthalmology with the University of Tokyo.
-
Reporting on Research Results
at International Conferences
Joint research with Juntendo University
Sato conducts research using molecular biology and genetic technologies. The research results we obtain are presented at domestic and international conferences, actively advancing basic research directly tied to drug discovery. We also utilize the knowledge acquired through basic research in the discovery, identification and validation of drug targets.
-
Publication in International
Academic Journals
Published paper
Based on findings from gene and protein analysis research, Sato has clarified the relationship between pathogenesis mechanisms and candidate target molecules; the results of that research were published in the international academic Journal of Dermatological Science.